
Consumers are being alerted to a voluntary recall of specific Robitussin cough syrup products over concerns of possible microbial contamination. The impacted products are feared to contain a microorganism that could potentially pose a risk to individuals with weakened immune systems.
Affected Products
The recall applies to the following Robitussin products:
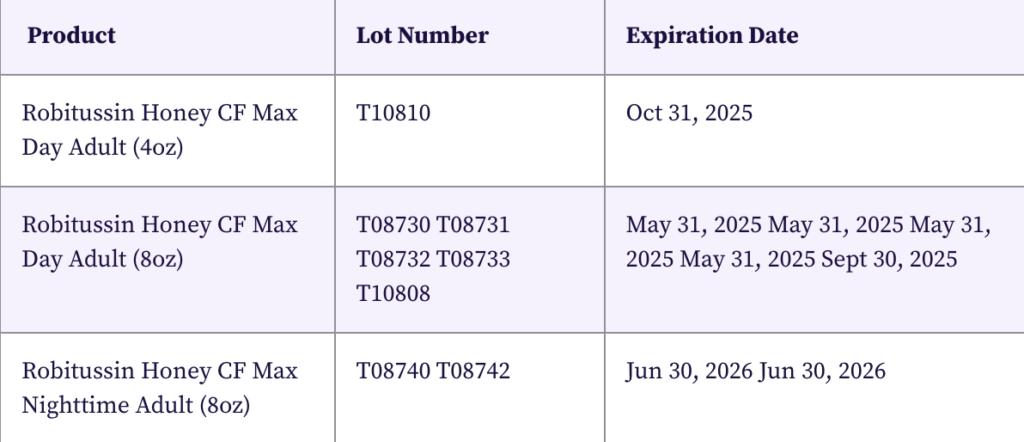
Customers are advised to look for specific lot numbers and expiration dates on their Robitussin packaging to determine if their purchase is included in the recall.
Health Risks
While the microorganism implicated in the recall is typically not dangerous to healthy individuals, there is a higher risk for those with severely compromised immune systems, such as individuals with HIV/AIDS, organ transplant recipients, or those undergoing certain types of treatment, such as chemotherapy.
Company Statement
The company responsible for Robitussin has issued a statement expressing their commitment to the highest quality standards and the safety of their consumers. They are working closely with regulatory authorities to ensure a swift and effective recall process.
Recommended Actions
Customers with affected products should:
- Stop using them immediately.
- Contact the company for instructions on how to receive a refund or replacement.
- Consult a healthcare professional if they have health concerns related to product use.
The recall serves as a precautionary measure to prevent any potential risks to consumer health. For the most accurate and up-to-date information, customers should visit the company’s website or the FDA’s recall page.
Contact Details for More Information
- Company Contact: Consumers can reach out directly to the customer service line specified on the product’s packaging.
- FDA Recall Page: Visit www.fda.gov for more information on this and other recent drug recalls.

