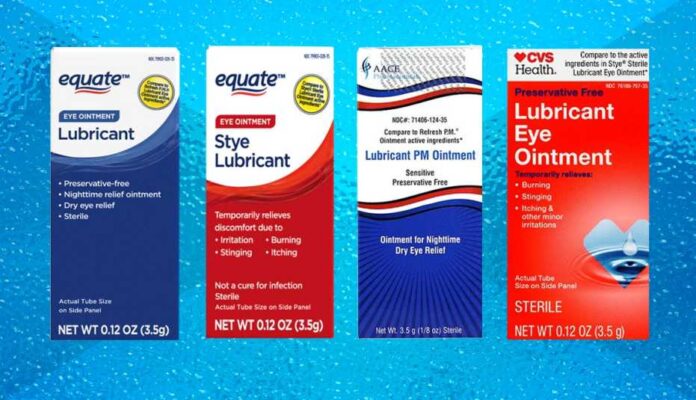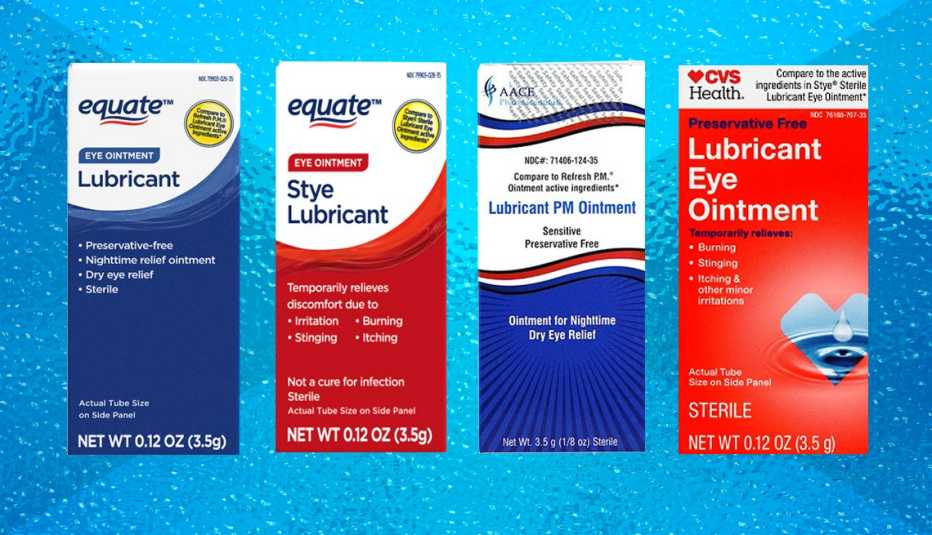
In a precautionary step to protect consumer health, specific batches of eye ointments available at prominent retailers such as Walmart and CVS have been recalled due to potential infection risks. This measure aims to prevent any possible complications that could arise from using these over-the-counter products.
What Triggered the Recall?
The recall comes after concerns were raised about the sterility of these products. Sterility in medical and ocular products is paramount to prevent microbial contamination that could lead to infections. An internal inspection found that some of the ointment batches did not meet all of the stringent guidelines required for sterility, prompting the recall.
Products Affected by the Recall
It’s important for consumers to check if they have purchased any of the recalled products. The specific items and batch numbers can be found on the FDA’s website or directly on the retailers’ notices. It typically includes various eye ointments that are used for treatments like dryness and irritation relief. Often, these products come in small tubes and are applied directly to the eye or eyelid.
Steps to Take if You Have the Recalled Ointments
- Stop Using the Product: If you possess any of the recalled eye ointments, cease using them immediately.
- Check the Batch Number: Compare the lot number on your product with the list provided by the recall notice.
- Return the Product: Return the ointment to the place of purchase. Many stores offer a refund or exchange for recalled items.
- Contact a Healthcare Provider: If you’ve used any of the recalled products and are concerned about your health, or if you have experienced any adverse reactions, you should contact your healthcare provider.
Implications for Health
Non-sterile eye products can pose a significant risk of eye infections, which can be particularly severe. These infections can cause symptoms such as eye pain, discomfort, redness, or even more severe conditions like ulcerative keratitis, which can threaten vision if not treated promptly.
Prevention and Caution
Consumers are advised to always check the seal and packaging for integrity before purchasing and using ocular products. Awareness of recalls and alerts issued by the FDA is also critical in ensuring the products used are safe and up to standards.
Moving Forward
Retailers like Walmart and CVS are working closely with the manufacturers to rectify the issue and hoping to resume the supply of safe and sterile eye ointments as soon as possible. The proactive response to potential health risks demonstrates the industry’s commitment to consumer safety.
Customers with affected products should monitor their health and report any adverse symptoms to their healthcare provider while following recall instructions to ensure their continued safety and wellness.
For more detailed information and specific guidance, visit the FDA’s website or contact the customer service department of the retailer where the product was purchased.